Your Cart is Empty
- Products
- CRISPR & RNAi Libraries
- CRISPR/RNAi Support Products
- Cas9 Cell Lines: Pre-made & Validated
- Custom Cas9 Cell Line Service
- CRISPR-Test Cas9 Activity Assays
- CRISPR sgRNA Cloning Vectors
- CRISPR sgRNA Control Constructs
- NGS Prep Kits for sgRNA / shRNA Libraries
- NGS Analysis Services for Libraries
- Bioinformatics Analysis of Screened Samples
- RNAi shRNA Control Constructs
- Cas9 and dCas9 Expression constructs
- LentiTrans Transduction Reagent
- CRISPR/RNAi Screening Services
- Cell Lines
- CloneTracker™ Cell Barcoding Libraries & Constructs
- Lentiviral Constructs
- Lentiviral Packaging
- Cloning Vectors
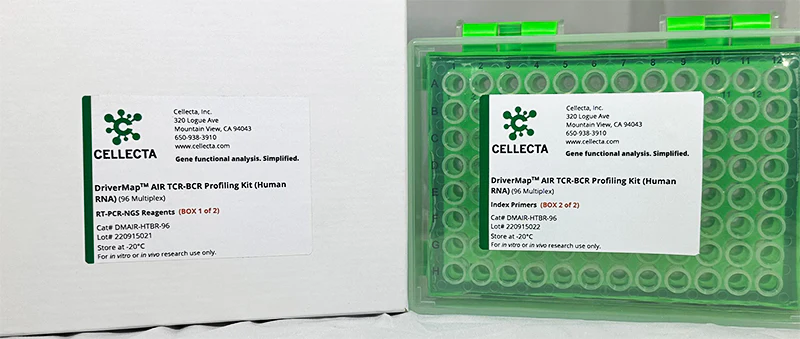
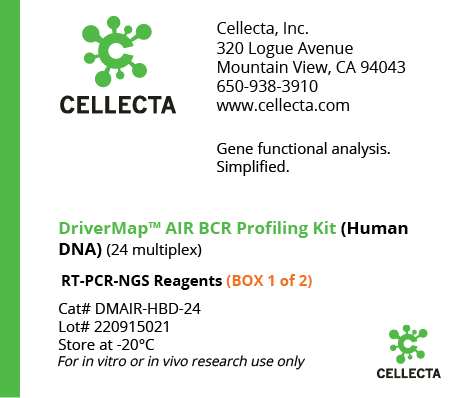
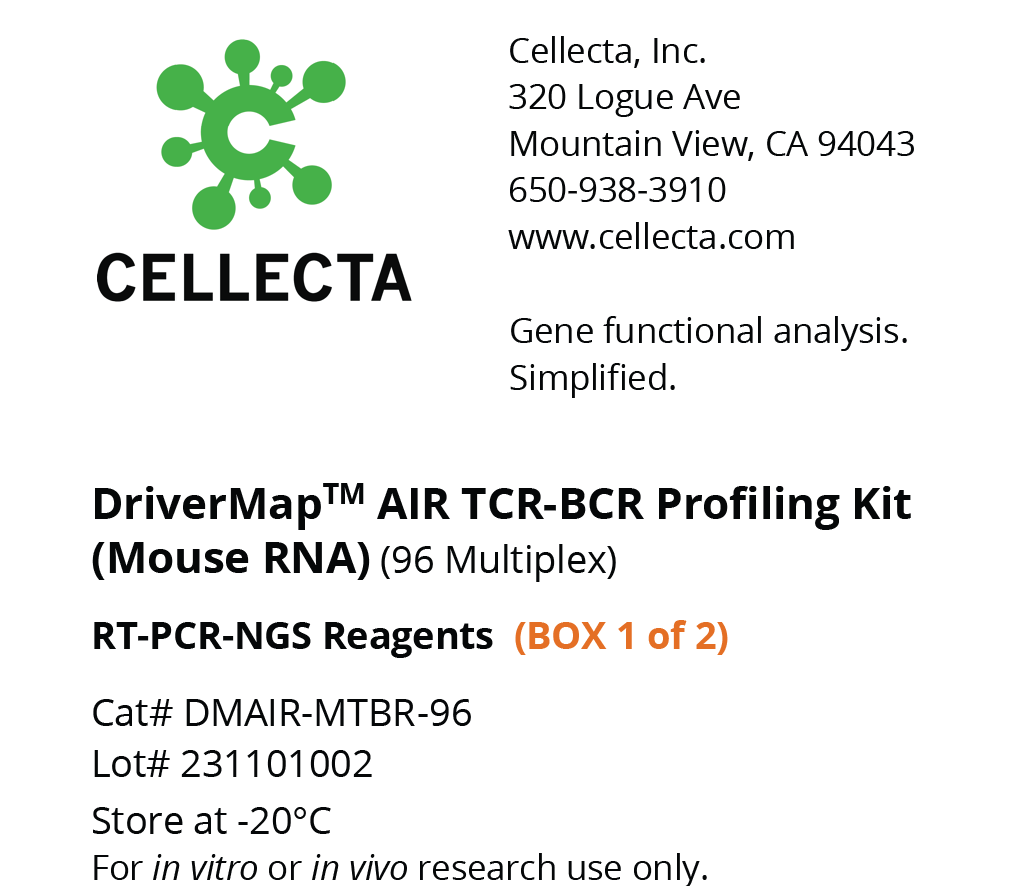
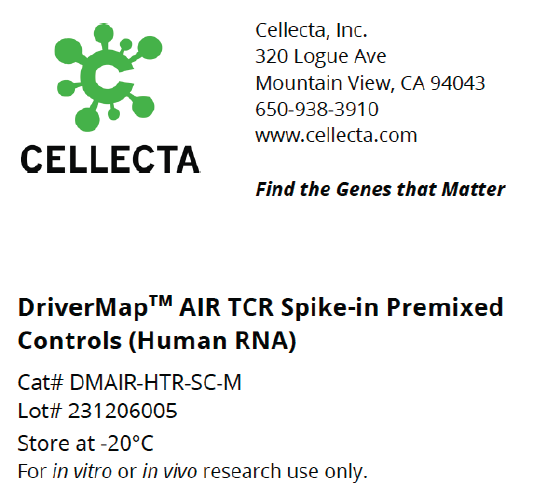
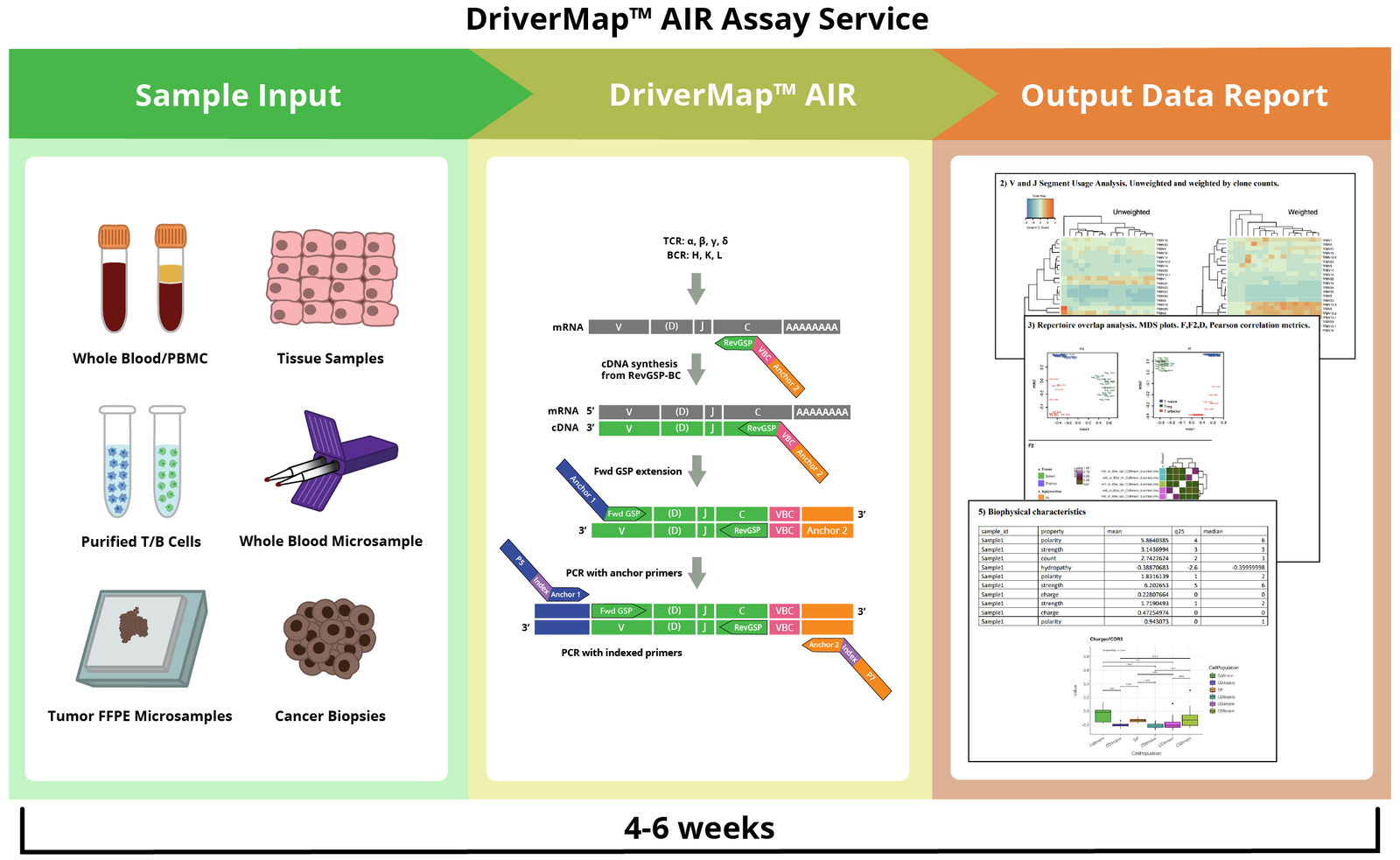
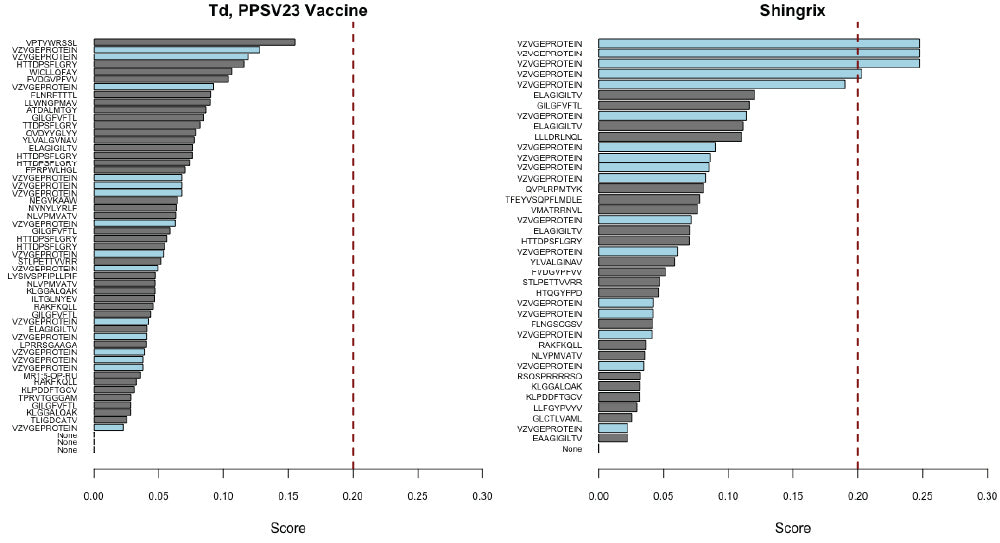
- DriverMap™ AIR TCR / BCR Repertoire Profiling
- DriverMap™ EXP Targeted RNA-Seq Expression Profiling
- Services
- Cell Engineering
- Custom Libraries
- Genetic Screens
- HLA Typing Service
- Lentiviral Constructs
- Lentiviral Packaging
- NGS of CRISPR/RNAi and Barcode Libraries
- TCR-BCR Repertoire Profiling
- Transcriptome Profiling
- Tissue-2-Target Service
- Bioinformatics Analysis Services
- DriverMap AIR / EXP Analysis Services
- AIR Adaptive Immune Receptor TCR / BCR Profiling Service
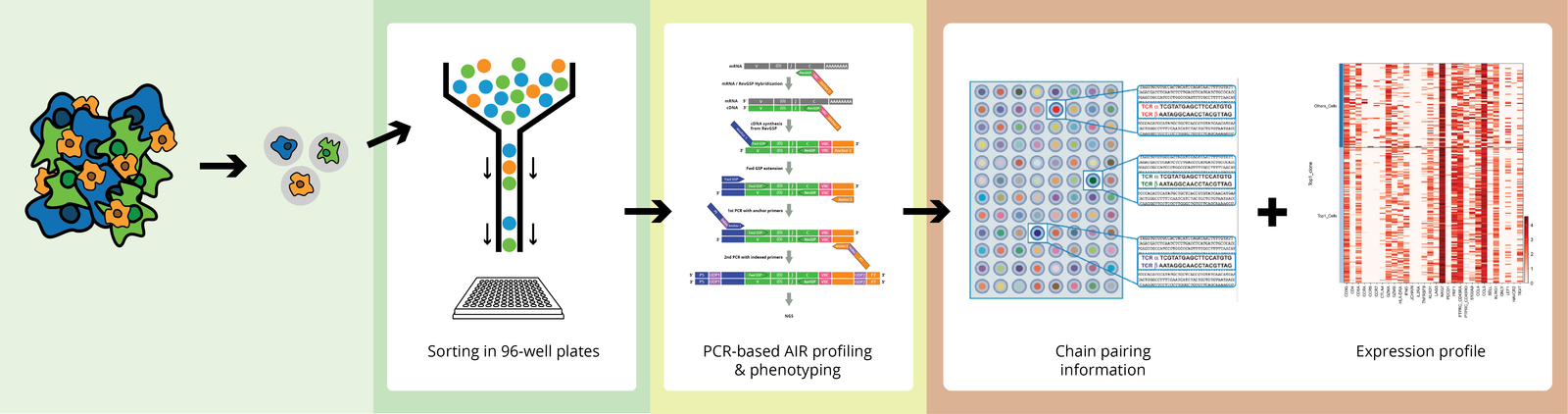
- Single-Cell AIR TCR / BCR Profiling Service
- Targeted RNA-Seq Expression Profiling (EXP) Service
- Custom Development of DriverMap™ EXP Assay
- Bioinformatics Analysis of DriverMap EXP or RNA-Seq data
- Bioinformatics Analysis of Bulk or Single-Cell AIR Profiling Assay
- Technology
- Resources
- Ordering
- Cas9 Cell Lines: Pre-made & Validated
- Custom Cas9 Cell Line Service
- CRISPR-Test Cas9 Activity Assays
- CRISPR sgRNA Cloning Vectors
- CRISPR sgRNA Control Constructs
- NGS Prep Kits for sgRNA / shRNA Libraries
- NGS Analysis Services for Libraries
- Bioinformatics Analysis of Screened Samples
- RNAi shRNA Control Constructs
- Cas9 and dCas9 Expression constructs
- LentiTrans Transduction Reagent
- AIR Adaptive Immune Receptor TCR / BCR Profiling Service
- Single-Cell AIR TCR / BCR Profiling Service
- Targeted RNA-Seq Expression Profiling (EXP) Service
- Custom Development of DriverMap™ EXP Assay
- Bioinformatics Analysis of DriverMap EXP or RNA-Seq data
- Bioinformatics Analysis of Bulk or Single-Cell AIR Profiling Assay